17 Mar 2021
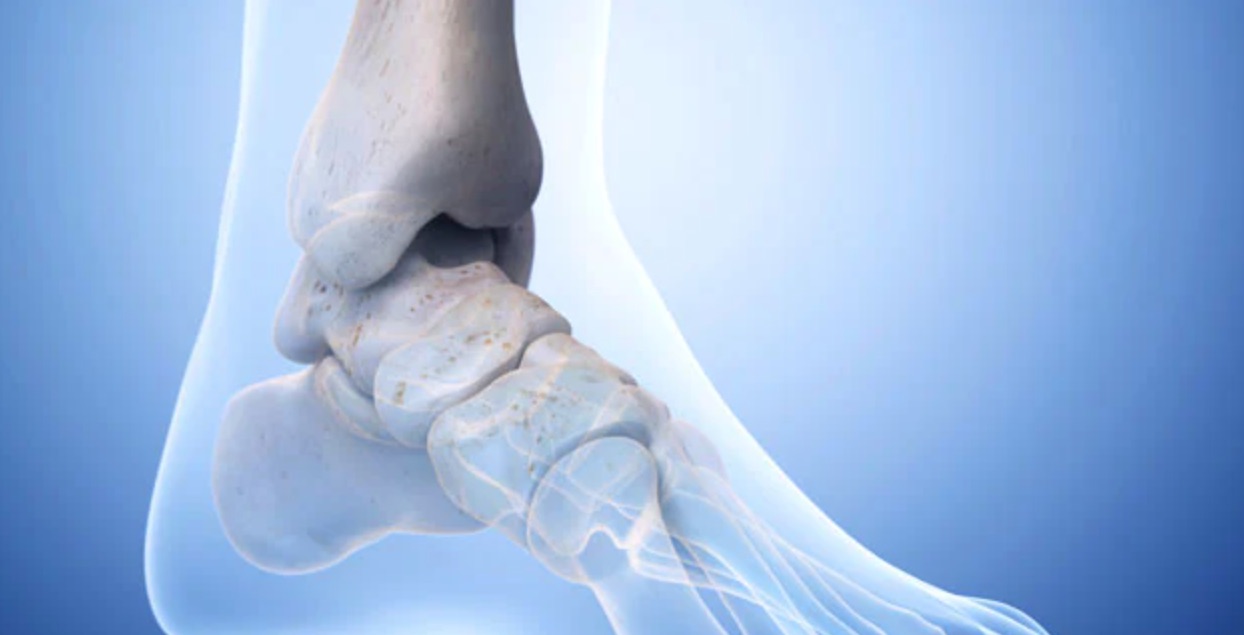
On March 15, 2021, the U.S. Food and Drug Administration announced that all Scandinavian Total Ankle Replacement (STAR) devices might fail prematurely, no matter when they were made or distributed. This warning updates previous warnings that only included devices made before 2014.
The STAR Ankle is one of the most implanted ankle devices in the United States. However, the FDA warns th...